Not only solutions determine the success but also implementation. We go that extra step in supporting and ensuring success for our customers. Providing formulation evaluation and recommendations for an optimal implementation. These are tailor-made services to ensure the best results. Areas include amino acid (AA) formulation, Metabolic Profile Evaluation and Environmentally Responsible Production (Nitrogen and CO2 Excretion, Carbon Footprint and Animal Welfare).
In our experience it is impossible to meet Lysine and Methionine requirements in mid-range crude protein diets. This also applies to both corn silage and grass silage fed herds with SBM and RSM (Table 1). When selecting the right rumen-protected amino acid, we have to choose the RPAA which provides the higher number of grams of metabolisable amino acid per unit price. We define metabolisable amino acid, as the amount of total ingredient in the product that can withstand the most aggressive mixing actions at industrial and farm level, also resisting rumen degradation enabling a higher release of Methionine or Lysine in the small intestine. This is the amount in which we formulate our diets and the amount the animal will use to achieve a predictable response.
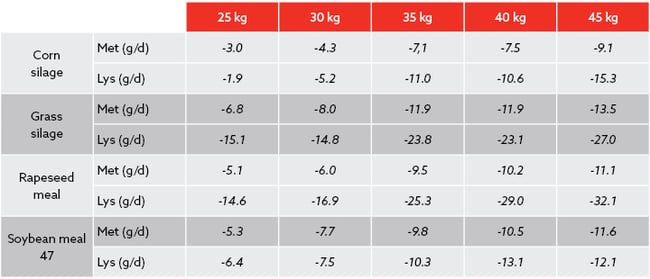
Table 1. Rough Lysine & Methionine deficiency in different diet milk production scenarios (from 25 to 45 kg). DIM=100, Kg M/DMI=1.4-1.5, CP=16-17 %, MF=3.8% & MP=3.3 %, Corn silage=30 DM-24NDF, Grass silage= 16 CP, 55 NDF. Own data, formulated according CNCPS v 6.55

The main reason why you don't achieve the desired result when balancing for amino acids is the overestimation of Methionine and/or Lysine that comes from the feed ingredients (including RP-Met and/or RP-Lys supplements). Another common problem is the underestimate of animal requirements, which equates to losing formulation efficiency and precision. As part of our Lifelong Learning Program, we have implemented the 5-step process for RPAA matrix validation. This methodology includes different processes for measuring ruminal degradation and post ruminal availability of Methionine and Lysine.
These processes are required in order to achieve a referenceable matrix validation:
Kemin rumen-protected amino acid products, KESSENT® (rumen-protected Methionine supplement range) and LysiGEM™ (rumen-protected Lysine supplement) are scientifically proven, consistently tested under field conditions, and supported through our Lifelong Learning Program.
Thanks to our Lifelong Learning Program, we all benefit from the 5-step process when using KESSENT and LysiGEM. Due to the results returned from these processes, certain predicted outcomes are assured: Methionine blood peak of 2h for our KESSENT MF range and 9 hours for KESSENT M. Lysine blood peak occurs at 6h post-feeding for LysiGEM. These values mean a very low rumen retention time, which translates to a high bioavailability, providing >50% of bioavailability for KESSENT MF, >80% bioavailability for KESSENT M and >70% bioavailability for LysiGEM. The 5-step process confirms excellent stability for our products under typical industrial and farming conditions, providing a high predictable response when implemented in ruminant dietary formulations.
With the right technical expertise and correct rumen-protected amino acids we can optimise success:
© Kemin Industries, Inc. and its group of companies 2026 all rights reserved. ® ™ Trademarks of Kemin Industries, Inc., USA
Certain statements may not be applicable in all geographical regions. Product labeling and associated claims may differ based upon government requirements.