Different technologies have been developed to protect Methionine and Lysine from microbial degradation, allowing the rumen-protected amino acids (RP-AA) to pass to the abomasum and small intestine where they are absorbed. However, producing products with both excellent protection from ruminal degradation and high intestinal release is not easy. Another critical point is the stability of those supplements in a feed matrix or within a total mixed ration.
A good RP-AA must survive the passage through the mouth (mastication, salivation), releasing little amino acid in the rumen and passes quickly into the omasum. The release will be for most of the RP-AA in the abomasum or proximal small intestine. Evaluating different methods to quantify ruminal AA escape and measuring ruminal outflows and their digestibility provides us a reliable characterization of the RP-AA matrix (what we call true bioavailability). Therefore, with these limitations, reducing the mean retention time in the rumen by varying the size and density of particles is a promising way to increase the rumen-undegraded protein and improve the effectiveness. If we increase the particle size of the RP-AA, the retention time increases (Welch and Smith, 1978; Welch, 1982; Ehle and Stern, 1986; Kaske and Engelhardt, 1990; Clauss et al., 2011) exponentially (Lechner-Doll et al. (1991)) or following a linear response curve (Dufreneix et al., 2019). Regarding the functional specific gravity, particles with densities of 1.2 to 1.3 had the lowest retention time in the entire digestive tract and in the rumen (Dufreneix et al., 2019). Even with the same technology protection, the difference in the core technology creates an important improvement in the intestinal bioavailability. Another important point is that an effective RP-AA, apart from to have high true bioavailability must be stable during grain mix preparation, feed mixing, and in the ration.
Thus, the key features of an optimal rumen-protected Methionine or Lysine are:
Accurate and precise measurement techniques are imperative for obtaining reliable experimental results on N and AA utilization. Therefore, at Kemin we developed our 5-step process for rumen-protected amino acid matrix validation.
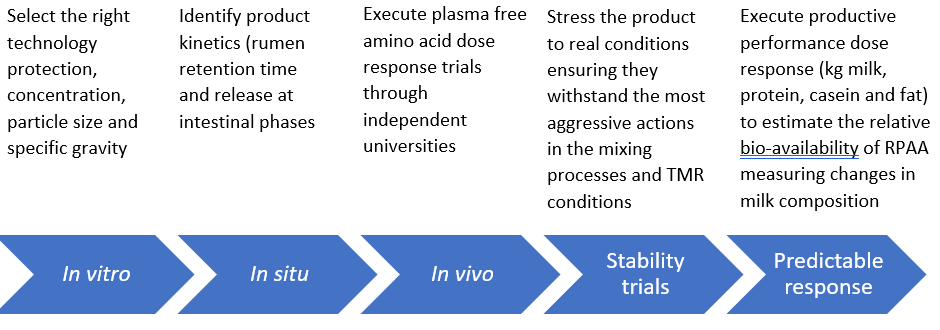
Knowing rumen protection rate and intestinal availability of the RP-AA supplement, gives us the primary AA available to the host animal (Hristov et al., 2019). Metabolizable AA delivered can be estimated using in situ, in vitro, or in vivo techniques with each having inherent advantages and disadvantages. The in vitro techniques may provide satisfactory accuracy for comparative purposes. While the in situ methodologies are widely used to estimate ruminal degradation of dry matter (DM) and CP. In vivo trials are the most logical and desirable way to evaluate the true bioavailability, like the plasma free AA dose response or Area Under Curve trials, but other techniques are needed if we want to assure the success of the amino acid implementation. Hence, we shouldn´t forget that another two extra trials are crucial if we want to work with the most reliable RP-Met or Lys supplements: the stability tests (for a viable RP-AA product the stability must be maintained in feeds and TMR), and the predictable response, to estimate the relative bio-availability of RPAA measuring changes in production, milk composition (Sameulson et al., 2001) or casein composition as the most influenced milk protein fraction.
Kemin ensures that in all its RP-AA products the matrix values are well defined and achieve all the goals for reaching the highest metabolizable AA in the ruminant. All RP-AA range produced by Kemin, KESSENT® M, KESSENT MF, LysiGEM™ or LysiPEARL™, provides a new protection grade with a high rumen protection rate and intestinal bioavailability together with a specific Kemin Core Technology. Its particle size and specific gravity, with a very low standard deviation, allows a high rumen scape with a very high intestinal bioavailability, because it was not needed to overprotect the nutritional additive. Likewise, its especial spheronization gives the product a higher stability at TMR conditions in the market after 24 hours of incubation. The high homogeneity of the product enables to achieve high quality of mixing in premixes and feed matrices too.
Our rumen-protected Methionine and Lysine are measured with in-vivo trials and are confirmed within our 5-step process for rumen-protected Amino Acid matrix validation. Our solutions provide reliable information in every one of the 5 steps with a reliable and high true bioavailability.
Read more about our rumen-protected amino acid product range here.
© Kemin Industries, Inc. and its group of companies 2026 all rights reserved. ® ™ Trademarks of Kemin Industries, Inc., USA
Certain statements may not be applicable in all geographical regions. Product labeling and associated claims may differ based upon government requirements.